XON 7
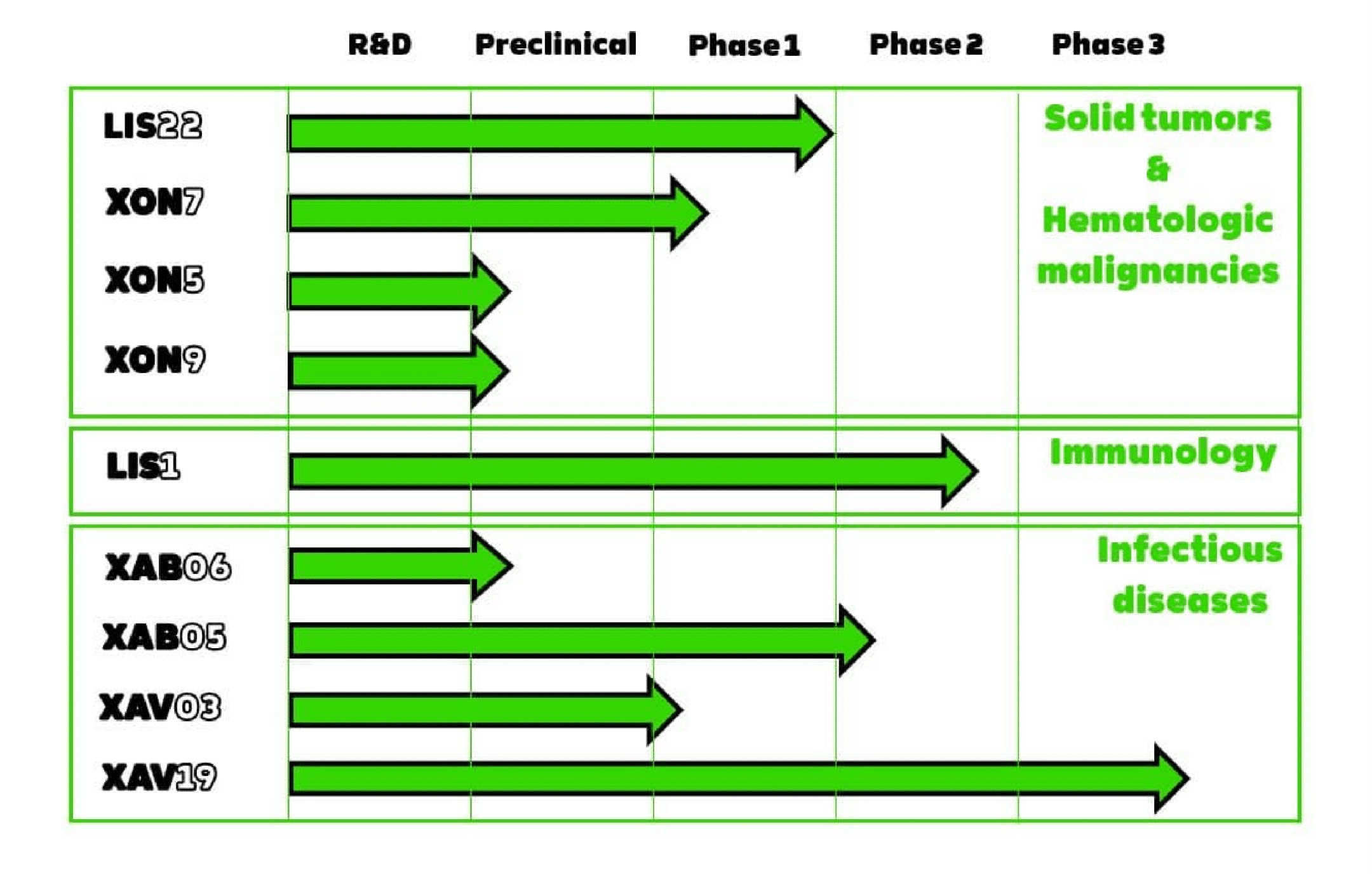
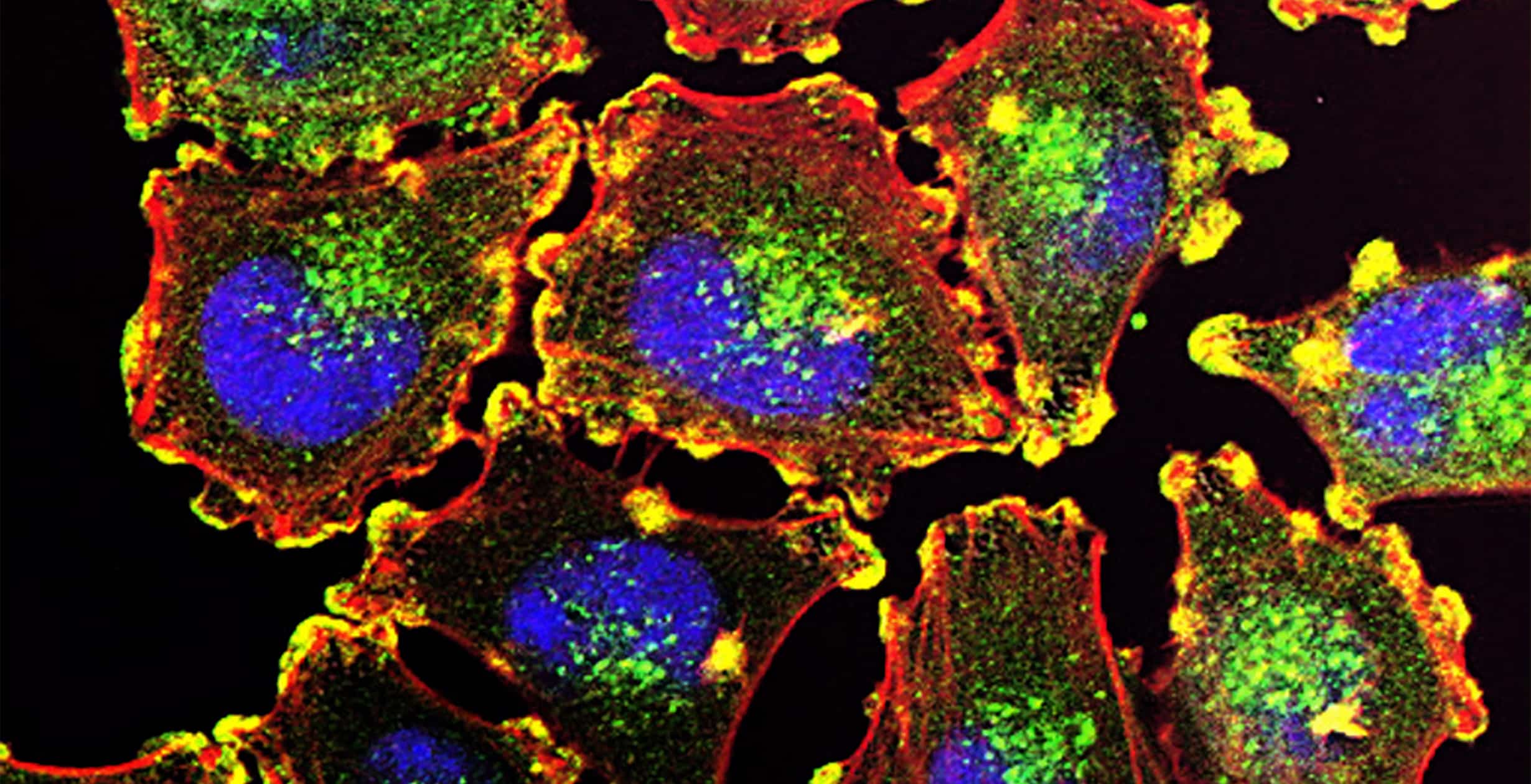
XON7 is a multispecific antibody issued from the GH-pAb platform by XENOTHERA. It targets several solid tumors including major cancers where the unmet medical need is important, namely lung, prostate, ovarian, pancreas. XON7 targets several tumoral antigens. Its biological activity is based on its specific mechanisms of action, namely CDC, apoptosis, ADCP and immunity induction. XON7 is in clinic since 2023.