Technology
Discover the different innovations that makes XENOTHERA an agile company with a fast-paced development.

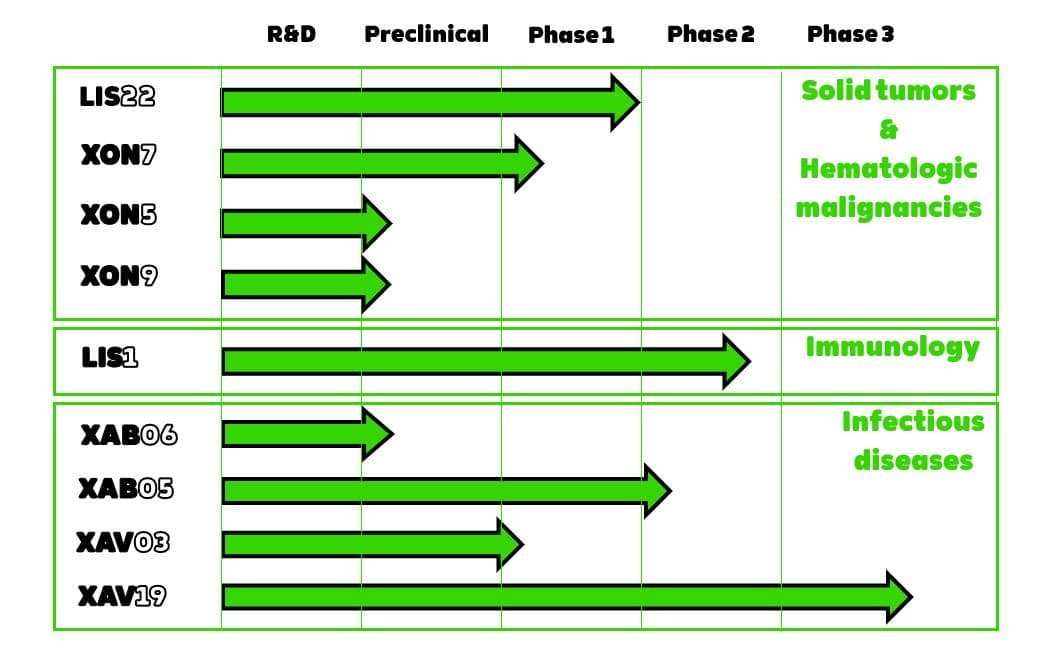
Clinical trials
Our policy is to allow any volunteering patient to participate in our clinical trials, if eligible.
Newsroom
XENOTHERA is built on a worldwide network of scientific & medical collaboration.